The interatomic spacing between the carbon atoms of graphite is a function of temperature. At 0 K, these atoms have their lowest energy position or ground state. The increased energy resulting from increasing temperature causes the atoms to vibrate and move further apart. In other words, the mean interatomic spacing increases and
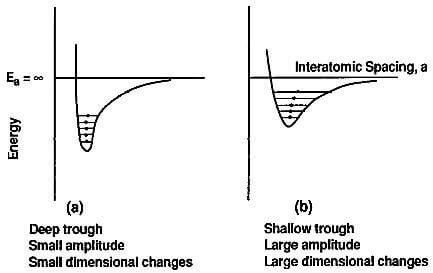
fig.3.11-the energy trough of graphite in (a)ab and (b)c direction
the result is thermal expansion.
This can be represented graphically in Fig.3.11. As seen in this figure, the graphic relationship between interatomic
spacing and energy has the configuration of a trough. This configuration changes with the strength of the atomic bond. In a strongly bonded solid such as graphite in the ab directions, the trough is deep, the amplitude of the vibrations is small and, during the outward motion of the atoms, the atomic bonds are not overstretched and, consequently, the dimensional changes remain small. When the atomic bond is weak such as in graphite in the c direction, the energy trough is shallow
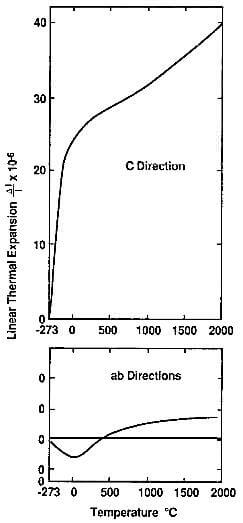
fig.3.12-thermal expansion of the graphite crystal in the ab and c directions.
and the vibration amplitude and the dimensional changes are large.
As a result, the thermal expansion of the graphite crystal has a marked anisotropy. It is low in the ab directions but higher by an order of magnitude in the c direction, as shown in fig.3.12.
The increase with temperature is not linear. In the c direction, it increases slowly and gradually. At 0C, the coefficient of thermal expansion averages 25×10-6/C and at 400C, it reaches 28×10-6/C.
In the ab directions, the thermal expansion is actually negative up to approximately 400C with a minimum at 0C. It is possible that this observed negative expansion is due to internal stress associated with the large expansion in the c direction and it has been suggested that, if it were possible to measure the ab thermal expansion of a single atomic plane, this expansion would be positive.
The large thermal expansion anisotropy often results in large internal stresses and structural problems such as delamination between planes as will be seen in other sectors.