The thermal properties of conductivity and expansion are strongly influenced by the anisotropy of the graphite crystal. The thermal conductivity (K) is the time rate of transfer of heat by conduction. In graphite, it occurs essentially by lattice vibration and is represented by the following relationship:
Eq (1) K=bCpvL
Where b= a constant
C= specific heat per unit volume of the crystal
v= speed of heat-transporting acoustic wave
L= mean free path for wave scattering
In a polycrystalline materials, the waves are scattered by crystallite boundaries, lattice defects, and other phonons.
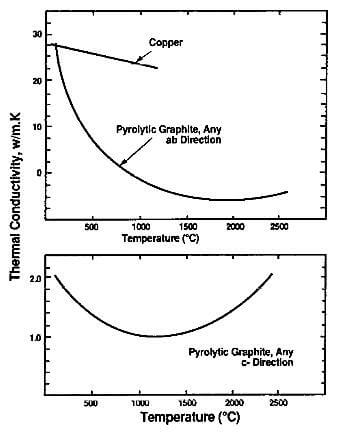
Fig.3.9-thermal conductivity of graphite crystal in the ab and c directions
Little of this occurs in a perfect or near-perfect graphite crystal in the basal plane and, as a result, the factor L is high and thermal conductivity is high in the ab directions. However, in the direction perpendicular to the basal plane (c direction), the conductivity is approximately 200 times lower since the amplitude of the lattice vibration in that direction is considerably lower than in the ab directions. These differences in vibration amplitude in the various crystallographic directions of graphite are shown in Fig.3.9.
The thermal conductivity of a graphite crystal has been reported as high as 4180 W/m.K in the ab directions for highly crystalline, stress-annealed pyrolytic graphite. However, the average value for commercial pyrolytic graphite is considerably smaller. Still, this is a high value and graphite, in the ab directions, can be considered a good thermal conductor comparable to high-conductivity metals and ceramics as shown in Table 3.6. Graphite fibers from pitch precursor have high thermal conductivity up to 1180 W/m.K, nearly three times that of copper.
Table 3.6. Thermal conductivity of selected materials
W/m.K at 25C
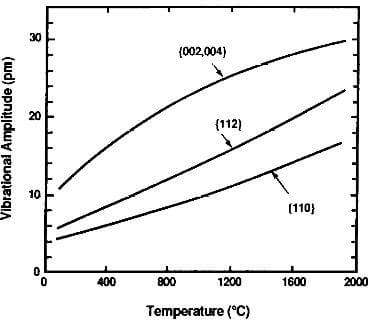
Fig.3.10-thermal vibrational amplitude of graphite crystal
Pyrolytic graphite:
ab directions 390
c direction 2
Graphite fiber (pitch-based) 1180
Diamond (Type Ⅱ) 2000-2100
Silver 420
Copper 385
Beryllium oxide 260
Aluminum nitride 200
Alumina 25
The thermal conductivity in the c direction is approximately 2.0 W/m.K and, in that direction, graphite is a good
thermal insulator, comparable to phenolic plastic.
The thermal conductivity of graphite decreases with temperature as shown in Fig.3.10. In the Debye equation, K is directly proportional to the mean free path, L, which is turn is inversely proportional to temperature due to the increase in vibration amplitude of the thermally excited carbon atoms. L becomes the dominant factor above room temperature, more than offsetting the increase in specific heat, Cp, shown in Fig. 3.8.