Graphite is an excellent refractory material with a melting point of about 4473C, but it must be under a pressure of some 100 atm, otherwise it just simply sublimes. Since graphite oxidizes slowly in air at about 400C, it should be protected in an inert atmosphere to operate successfully at elevated temperatures and, it is most important that the inert atmosphere at temperatures in region of 2500C is quiescent, since otherwise, the surface will be badly eroded at the gas flow sweeping across the surface continually removes the surface graphite by sublimation.
Specific heat increases with temperature (Fig 2.24) and whilst graphite is considered a good conductor in the
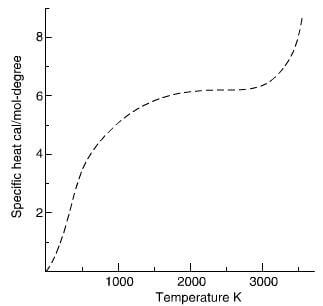
schematic plot of the specific heat of graphite
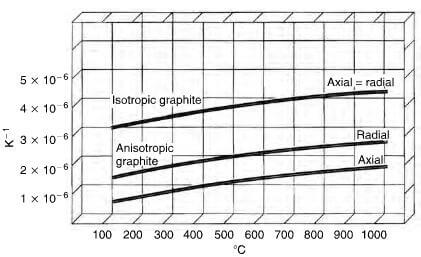
thermal expansion of anisotropic and isotropic graphite
basal direction, it is highly anisotropic and is almost an insulator in the direction normal to the basal plane. The thermal conductivity decrease with temperature. The thermal expansion of an extruded rod in a radial direction can be up to three times the value in an axial direction (Fig 2.25) and due care must be taken to accommodate this anisotropy in practical applications.
Graphite is a good conductor of electricity as well as heat and again, is extremely anisotropic, behaving like a metal in the direction of the basal plane and virtually a non-conductor normal to it. In practice, however, molded graphite is quite different in its behavior due to the effect of the different fabrication routes, which can affect the direction of grains, the actual grain size, the porosity and the finished size. The electrical resistivity is an important parameter for graphite when used for the construction of heating elements. Fig2.26 shows the effect of temperature on a typical electrode material, whilst Fig2.27 shows the effect of the type of graphite and direction of grain on the electrical resistivity.
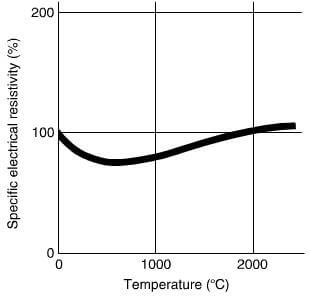
relative change in the specific electrical resistivity of graphite with temperatures.
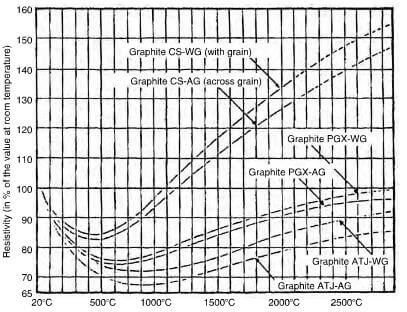
variation of the resistivity at high temperature of various types of graphite. CS-extruded, PGS-molded, ATJ-fine grain.