The ideal hexagonal graphite structure described above is composed of theoretically infinite basal planes with perfect –ABAB- stacking, with no defects whatsoever. Such an ideal structure is, of course, never found, either in natural or synthetic graphite.
Crystallite imperfections: Within each crystallite, a varying number of imperfections may be found as shown in
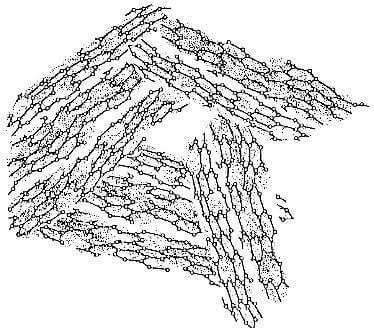
fig.3.4-structure of turbostratic graphite. note lattice defects and vacancies.
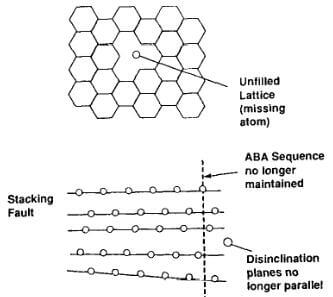
Fig.3.5-schematic of crystallite imperfections in graphite
figs. 3.4 and 3.5. These include:
- Vacancies, when lattice sites are unfilled indicating a missing atom within a basal plane
- Stacking faults when the ABAB sequence of the layers planes is no longer maintained
- Disclination when the planes are no longer perfectly parallel
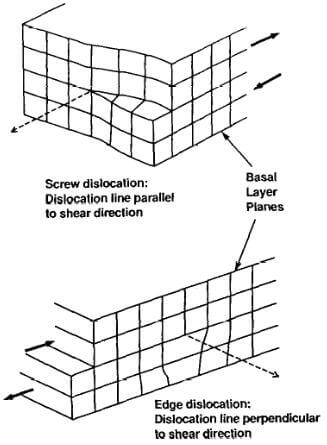
Fig.3.6-shear dislocations in a graphite crystal
Other crystalline imperfections likely caused by growth defects are screw dislocations and edge dislocations (Fig. 3.6). The presence of these imperfections may have a considerable influence on the properties of the bulk material.
Thus in each graphitic material, the size, shape, and degree of imperfection of the basic crystallite, the general orientation of these crystallites, as well as the bulk characteristics such as porosity and amount of impurities, may vary considerably from one materials to another. As a result, the properties of these various materials may show considerable differences.
An important implication is that, whereas material differences in the various carbon materials were originally ascribed to the presence of an “amorphous” component, a more realistic approach is to relate these differences to the size and orientation of the graphite crystallites.
The specific structure and properties of the different graphitic materials will be reviewed in detail in subsequent chapters.