The ideal hexagonal graphite structure described above is composed of theoretically infinite basal planes with perfect –ABAB- stacking, with no defects whatsoever. Such an ideal structure is, of course, never found, either in natural or synthetic graphite.
Polycrystalline graphite: graphite materials, such as porolytic graphite, carbon fiber carbon matrix composites (carbon-carbon), vitreous carbon, carbon black, and many others, are actually aggregates of graphite crystallites, in other words, polycrystalline graphites. These crystallites may vary considerably in size. For instance, the apparent crystallite size perpendicular to the layer planes (Lc) of some vitreous carbons may be as small as 1.2 nm which is the length of a few atoms, or up to 100nm found in highly ordered pyrolytic graphites. The layer planes may or may not be perfectly parallel to each other, depending whether the material is graphitic or non-graphitic carbon.
The aggregates of crystallites also have widely different sizes and properties. Some, such as soot, are extremely
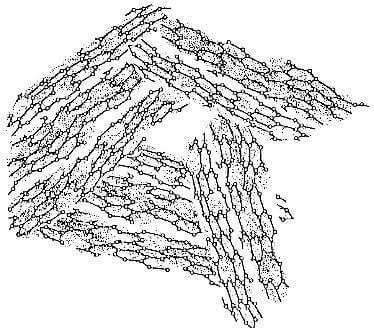
Fig 3.4-structure of turbostratic graphite. note lattice defects and vacancies.
small and contain only a few small crystallites. In such cases, the properties are mostly related to the surface area.
Other aggregates may be relatively large and free of defects and essentially parallel to each other, in which case the structure and its properties closely match those of the ideal graphite crystal. Such large aggregates are often found in porolytic graphite.
In other aggregates, the crystallites have an essentially random orientation. This occurs in turbostratic or amorphous carbon shown in Fig.3.4. In such cases, the bulk properties are essentially isotropic.