Gases are evolved from within the carbon-carbon composite during the oxidation process, mainly arising from active sites on the edges of layer planes and vacant sites formed by dislocations in the basal planes. These sites can be blocked or poisoned and the composite porosity reduced by using inhibitors based on B, P and halogen compounds which lower the reactivity of C with O2. The choice of inhibitor is limited by the temperature of application.
- Boron
The compound B2O3 is widely used for protection up to 1500C through poisoning sites as well as acting as a glassy sealant. It is a good choice, melting at about 450C. Although in use it is limited to about 1000C due to its volatility, as a crack sealant, the working temperature can be extended to 1200C and to about 1400C if coated with an outermost layer of SiC. The glass former migrates to the outer surface, sealing cracks and voids en route. At 1525C, the CO liberated as a result of the B2O3 reacting with the carbon-carbon surface
2B2O3+7C→B4C+6CO
Causes the partial pressure generated to disrupt the glass. Coupled with corrosion of SiC by th borate glass, the maximum temperature of use is limited to 1500C for short periods. TiO2 is soluble in B2O3, thus increasing the viscosity and helping to prevent volatilization. Boron coating are susceptible to attack by water at ambient temperatures, forming HBO2.
Figure 14.21 shows how boron can be absorbed at vacant edge sites of graphite crystallites and whilst a 3.5% B2O3 addition will effect a significant reduction in oxidation, 1 10% addition will reduce the oxidation rate by an order of magnitude, but increase the overall weight of the composite.
- Phosphorus
Organophosphorus compounds, when heated to 400C, forms a phosphate residue that is tenaciously held on the carbon surface, acting as a poison, blocking active sites and reducting the rate of oxidation up to working temperatures of 850-900C. This temperature can be extended when phosphates are used in conjunction with other inhibitors like B2O3, SiO2 and SiC. Phosphates are not as effective as borates.
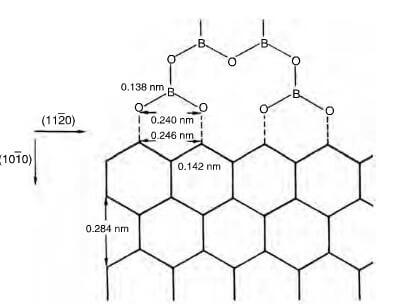
schematic representation of bonding of (BO3)n polymer to (1010) face of graphite lattice.