There is good experimental evidence that the properties of carbon allotropes or compounds are affected by the isotopic composition of the carbon atoms, as shown by the following examples.
One process for synthesizing diamond uses methane in which the carbon atom is a carbon-12 isotope enriched to 99.97% 12C. In this process, diamond is deposited by chemical vapor deposition (CVD) in a microwave plasma. The resulting 12C diamond is reported to have a thermal conductivity 50% higher than that of natural diamond which has the normal ratio of 12C and 13C of about 100/1.
The other example is related to the natural process of photosynthesis in organic matter. Photosynthesis is isotope-selective, i.e., less 13C is absorbed proportionally so that the carbon from organic sources is slightly poorer in 13C than inorganic carbon.
This selective absorption is important in geochemical studies since measuring the amount of 13C provides a good evidence of the origin of the carbon. For instance, the mineral calcite, which is a limestone composed mostly of calcium carbonate (CaCO3) found in the cap rock of salt domes, is low in 13C compared to most other limestones which have an inorganic origin. This indicates that the carbon in the calcite came from petroleum, rather than from non-organic sources.
Most limestones are formed from the bicarbonate ion of sea water, HCO3-, (which in turn comes from atmospheric CO2) and have the normal 13C content. The salt-dome calcite, on the other hand, is formed by the combination of a calciumion, Ca++, and the CO2 resulting from the oxidation of petroleum (hence from an organic source with less 13C. These formation processes are shown schematically in Fig.2.5)
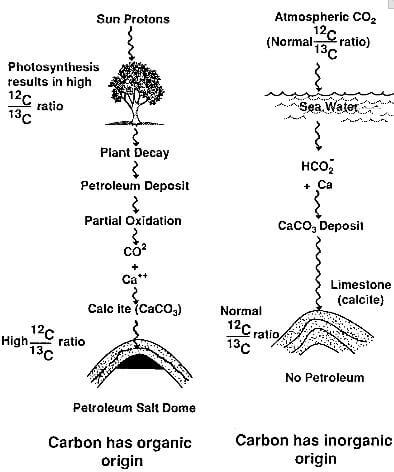
fig.2.5-organic origin of carbon in petroleum salt domes