The triple point is achieved, by recent estimates, at a temperature of 4200K and a pressure of 100atm, as shown in the vapor-pressure curve of Fig.3.7. A great deal of uncertainty still remains regarding these values of pressure and temperature, reflecting in part the difficulty of conducting experiments under such extreme conditions.
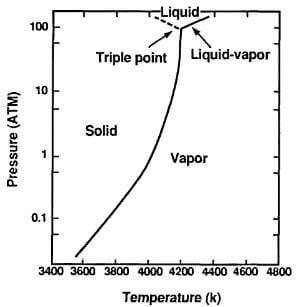
Fig.3.7-vapor pressure and triple point of graphite
The onset of sublimation and the melting temperatures are apparently close. At temperatures above that of the triple point and at pressures of argon greater than 100 atm, a mixture of solid and liquid carbon is detected.
Graphite can thus be considered the most refractory of all the elements, tungsten being second-best with a melting point of 3680K. However hafnium carbide and tantalum carbide are reported to have higher melting points and are the most refractory of all materials.
Heat of vaporization: The heat of vaporization of graphite is higher than that of many metals as shown in Table 3.3. Reported values show considerable differences.
The large amount of energy required to vaporize graphite is used to good advantage in the design of ablative structures such as nose cones and rocket nozzles.
Table 3.3 Heat of vaporization of selected elements at boiling point
kJ/mol
Graphite 355.8-598.2
Molybdenum 598.0
Copper 300.3
Iron 349.6
Nickel 370.4
Tungsten 824.0
Silver 250.5