2D C/C composite oxidation occurs preferentially at the fiber/matrix interfaces. This is in line with mismatch of coefficient of thermal
carbon fiber composite plates sheets U L profiles-2D material manufactuer china (5)
expansion (CTE) of the fiber and matrix which resulted in interfacial debonding. The gaps formed by interfaces oxidation provide a path for the oxygen attack, which can accelerate oxidation. Because of a high preferred orientation of crystallites with the 2D chain structure running parallel to the fiber axes, the interior of fibers is more oxidation resistant than the edges. As a result, the carbon fibers become thin and their ends become sharp with continued oxidation.
In the model-free method, the linear correlation is better (r>0.998). Hence, it is suggested that that the oxidation mechanism should be the same at identical α for different temperatures in each temperature range.As can be seen from fig.7, the apparent activation energy strongly depends on weight loss and temperature ranges. The comprehensive profound studies on the variation of activation energy with the temperature and the extent of conversion were done by Vyazovkin et al. According to the Vyazovkin’s theory, the oxidation of 2D C/C composite should be complex process involving two or more single-step processes; the variation of activation energy with weight loss and temperature ranges is attributed to the relative contribution of each single-step process, and the reflects the oxidation mechanisms change. So the oxidation mechanisms at lower temperatures may be different from that at higher temperatures, and the oxidation mechanisms may change below and above 60% burn off at higher temperatures.
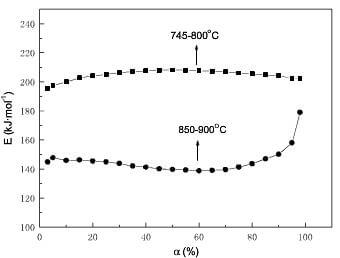
fig.7-dependence of Ex on a for 2D CC composite by model-free method
At lower temperatures, the value of apparent activation energy obtained by model-free method is relatively high, and the controlling mechanism is R2 by model-fitting and reduced-time plot methods. Hence, the oxidation is only controlled by surface reaction between carbon and oxygen in two dimensions in the whole process. The slight change of the athe difference of Ex at lower temp and higher temp before 60% burn off by model-free methodpparent activation energy obtained by model-free method may be due to the difference in reactivity of matrix and fibers.
At higher temperatures, the chemical reaction rate is higher, and the inside of composite is filled with product (CO, CO2). Oxygen must diffuse through the gas layer to react with carbon, and the transfer of reactant and product plays an important role for oxidation. The oxidation is controlled by chemical reaction and gaseous diffusion, which results in the decrease of the apparent activation energy. Examination of Fig.8 suggests the contribution of gaseous diffusion to oxidation gradually increase before 60% weight loss. However, the controlling mechanism is only R2 by model-fitting and reduced-time plot methods at α<60%. The reason may be that gaseous diffusion is macroscopic phenomenon, and the model-fitting and reduced-time plot methods are failed for the process controlled by macroscopic phenomenon and microcosmic mechanisms together. The model-free method succeeds in aforementioned process. When weight loss is over 60%, the amount of product reduces due to the sharp decrease of the oxidation rate. Meanwhile, the inside voids of composite enlarge with oxidation of matrix and the exterior of fibers. So the contribution of gaseous diffusion to oxidation decreases. But the chemical reaction rate is much higher than that at the lower temperatures. The remaining active sites which could easily react with oxygen cannot afford to maintain the reaction between carbon and oxygen in such high oxidation rate. In order to continue oxidation, it must be fulfilled that the fibers need to produce adequate active sites. Therefore, the random nucleation in two dimensions which assumes that there is a stochastic distribution of the potential transformation centers transforming into active sites, gradually plays an important role. So the controlling mechanisms of oxidation are R2, A2 and gaseous diffusion for α>60% at higher temperatures. It is found that the reduced-time plot method succeeds in the process controlled by one or two microcosmic mechanisms.
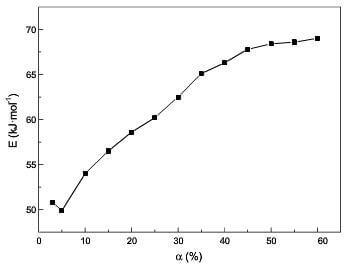
fig.8-the difference of Ex at lower temp and higher temp before 60% burn off by model-free method.
In the difference of Ex at lower temp and higher temp before 60% burn off by model-free method.2(a) and (b), the trend of oxidation rate with weight loss is same during the initial and final stage in the range 745-900°C; however, during middle stage, it has a dramatic difference between higher temperatures and lower temperatures. Now, let us analyze the reason why the oxidation rate has such trend with weight loss or temperature ranges by the combination of microstructure and mechanisms of oxidation.
In the initial stage, due to a mass of pits in possession of more active sites existing at the fiber/matrix interfaces, the oxidation rate is higher. With oxidation of the interfaces, the oxidation rate decreases. Though a mass of active sites were consumed with oxidation, the active sites can be regenerated by chemical reaction. At lower temperatures, the oxidation rate is controlled by chemical reaction, so the oxidation rate decreases with a slow rate from 10% to 40%. At higher temperatures, the oxidation rate depends on the gaseous transport rate during the middle stage, so the oxidation rate is in stable state till 60% is burn off. In the final stage of oxidation, the decrease of the diameter of carbon fibers and their surface micro-depressions shows the reduction of reaction surface area. Therefore, the oxidation rate decreases.
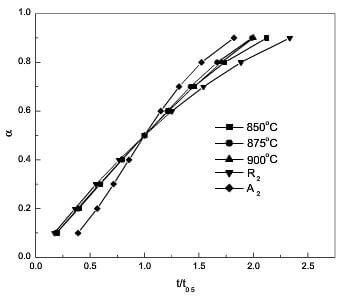
fig.9-Experimental a- tt0.5 curves and theoretical a -tt0.5 curves according to R2 and A2
Based upon these studies, a number of conclusions can be drawn about the oxidation kinetics and mechanisms of 2D C/C composite.
- Oxidation starts from the fiber/matrix interfaces, and matrix carbon is oxidized much more rapidly than the carbon fibers. With continued oxidation, the fibers exhibit reduced diameter, their ends become sharp, and their surface becomes glossy. In the final stage of oxidation, only the fibers are being attacked.
- The model-free curve is not a single straight line but consists of two straight lines, and the broken point is at about 800-850°
- At lower temperatures (745-800°C), the oxidation is controlled by surface reaction between carbon and oxygen in two dimensions (R2), and the corresponding apparent activation energy is about 195-208 kJ/mol.
- At higher temperatures (850-900°C), when weight loss is less than 60%, the oxidation is controlled by R2 and gaseous diffusion, and the contribution of gaseous diffuse to oxidation gradually increases. When weight loss is over 60%, the oxidation is controlled by R2, gaseous diffusion and random nucleation in two dimensions (A2).
related news /articles:
Oxidation kinetics and mechanisms of a 2D C/C composite (1)
Oxidation kinetics and mechanisms of a 2D C/C composite (3)
Introduction of high-temperature coatings on CC composite material
Catalytic oxidation of C-C composites & kinetics and mechanism of catalysis