Results of studying the perfection of material crystal structure of high-modulus fibers based on polyacrylonitrile with a carbon matrix of phenol formaldehyde resin coke in the production and treatment temperature range from 900 to 2500C are provided in Table 1. The three-dimensional crystallization (graphitization) of the test CCCM commences not sooner than reaching a temperature in the range 2100-2300C. The results obtained due to the fact that both components of the carbon composite of this production stage are related to “non-graphitized” components. Therefore a reduction in specific electrical conductivity of a carbon composite may be connected with transformation of the pore structure, compaction of contacts at the inter faces of a carbon matrix- carbon filler, and carbon matrix – carbon matrix.
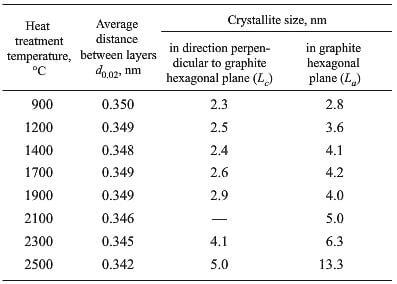
change in x-ray structural properties of carbon-carbon material of high-modulus threads based on polyacrylonitrile with HTT
In order to clarify the mechanism of the process activation Arrhenius activation energy was evaluated. The molecular rebuilding of a carbon substance should show an activation energy commensurate with the energy of the C-C bond. Diffusion processes have activation energy an order of magnitude less. In this case evaluation of activation energy of the change in material specific electrical conductivity in the treatment range from 600-1500C gives a value close to (38±2)kJ/mole. Previously a similar order of the value activation energy was found for the dependence of the change in amount of material, shrinkage through a specimen thickness during carbonization. For the dependence of the change in shrinkage rate for carbon material during carbonization in the period of most intense chemical transformations the level of activation energy found is ~20 kJ/mole. For the test carbonized material shrinkage was studied separately through the thickness of a specimen in the temperature range after completion of the main chemical transformations during 960 sec, until geometric dimension relaxation was complete. Here the level of activation energy found was 12.0 kJ/mole. These levels, markedly less than that for the level of chemical bond energy in carbon materials, indicating a relatively weak connection of the level of the degree of transformation with temperature, correspond to the kinetics of diffusion, and volumetric relaxation processes in a solid. The difference is levels from 12 to 38 kJ/mole may be connected with a change in plastic deformation resistance of the carbon matrix at the start and completion of carbonization. Thus, with a temperature up to 1200C in forming the level of specific electrical resistance there is predominance of compaction of the carbon material due to shrinkage. Shrinkage in turn, due to a difference in deformation properties of the matrix and filler, generates internal stresses at a microlevel. The relative slow down in reduction of specific electrical conductivity to the range 1300-1400C and its almost unchanged nature about up to 1400C point to completion of the individual stage of carbon matrix structure formation.
Starting with a treatment temperate of about 1500C there is a resumption of an increase in open porosity. An increase in porosity follows a resumption in weight loss, and also coincides with intensification of shrinkage, which is reflected in material compaction. It may be seen from the dependences presented with a change in open porosity, weight loss, and shrinkage, that the absolute intensity of physicochemical processes during carbonization markedly exceeds the rate of change in the stage of polymer binder hardening and up to the start of its thermal destruction.
In practice, in performing HTT at about from 1560 to 1680C in electric vacuum furnaces in this temperature range in some cases an increase is recorded in absolute pressure from 3.6 to 90 kPa. Here in the working production regimes there is a reduction in heating in order to provide the capacity of vacuum pumps to restore the original absolute pressure. Only then is heating resumed for the product being treated. On the whole however, weight loss in this temperature range is relatively small, and corresponds to 4.0-4.7%. This amount is less by almost a factor of five than weight loss during the preceding carbonization.
Strength in bending test results are shown in Fig. 4 for specimens in relation to temperature. At 1300-1400C
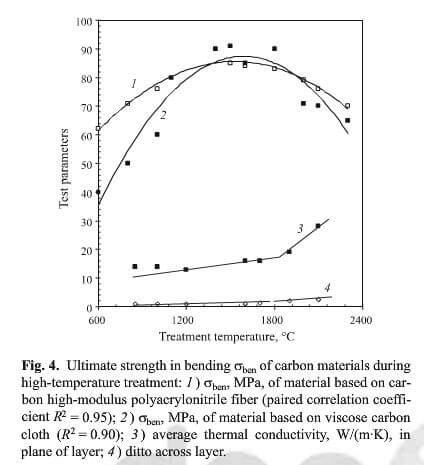
ultimate strength in bending of carbon materials during high-temperature treatment.
the relatively greatest level of strength of carbonized material is established. Experimental results have a maximum at 1400C. Strength exceeds by almost a factor of two that of material in the stage of carbon coke formation from pyrolyzed binder. The relative increase in mechanical strength of a carbon composite may be consequence of matrix shrinkage and compaction noted above. At 1300C, as is well known, apparent and true density of “glassy carbon” are at a maximum, i.e., industrial carbon material represented by phenol formaldehyde resin coke.
It follows from data in Figs. 3 and 4 that the temperature range for high-temperature treatment from 1270C to ~1500C for carbon-carbon composites of the composition and structure indicated has a range of steady-state condition. A composite in this condition may have commercial value if its operating temperature does not exceed the upper level of this range. On the basis of data in Figs. 1 and 2 proceeding from the calculated level of error, it is desirable to designate the production temperature for treatment from (1270+50) to (1500-50)C.