Fluidized-bed CVD is a special technique which is used primarily in coating particles such as nuclear fuel. A flowing gas imparts quasi-fluid properties to the particles. Fig.7.3 shows a typical fluidized-bed CVD reactor.
The fluidizing gas is usually mechane, helium, or another non-reactive gas. Factors to consider to obtain proper fluidization are the density and size of the particles to be coated, and the velocity, density, and viscosity of the gases. If the velocity is too low, the particles will fall into the gas inlet; if it is too high, they will be blown out of the bed. Heavy or large objects may require suspension in the bed.
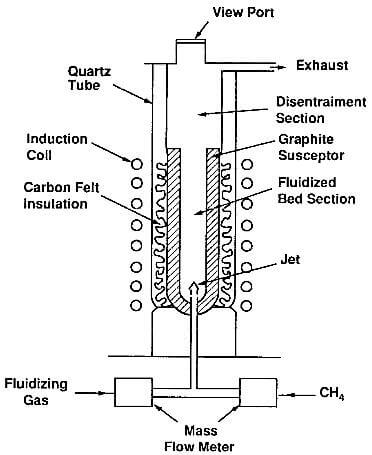
fig.7.3-schematic of a fluidized-bed reactor for the deposition of pyrolytic graphite
The gas velocity, Vm, is given by the following relationship:
Vm=d2(ρp-ρg)G/1650μ [for dVoρg/μ<20]
Where: d=particle diameter
ρp=particle density
ρg=gas density
G=acceleration of gravity
Vo=superficial gas vlocity
μ=gas viscosity
The major applications of pyrolytic carbon deposited by fluidized bed are found in the production of biomedical components such as heart valves and in the coating of uranium carbide and thorium carbide nuclear-fuel particles for high temperature gas-cooled reactors, for the purpose of containing the products of nuclear fission. The carbon is obtained from the decomposition of propane or propylene at 1350C, or of methane at 1800C. Its structure is usually isotropic.
Plasma CVD: The deposition of graphite can also be obtained by plasma CVD, with the following characteristics:
– Gases: proppylene-argon or methane-argon
– Plasma: radio frequency at 0.5 MHz
– Pressure: <1300Pa
– Temperature: 300-500C
In a plasma-activated reaction, the substrate temperature can be considerably lower than in thermal CVD. This allows the coating of thermally sensitive materials. The characteristics, and properties of the coating are similar to those of coatings deposited at higher temperatures.
Plasma activation is also used extensively in the deposition of polycrystalline diamond and diamond-like carbon. It is reviewed in more details in other articles.