Catalytic corrosion and role of residual impurities: Residual impurities resulting from C/C processing, for example CVD or impregnation, can also pose a concern in the operating environment. Metallic impurities, specifically transition metals, have been shown to exhibit catalytic activity in the gasification of carbon materials. The gasification rates increase with increasing concentration of impurities. The effect is significant only if the amount of catalysts is sufficient. The small amount of impurities present may not play an important role in corrosion, but may allow transport of metallic impurities of refractory metals forming brittle intermetallics. The amount and types of impurities will differ for each C/C vendor, and the role of residual impurities must be further investigated through experimental data.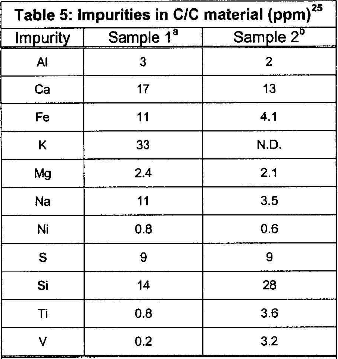
Compatibility with fission products: Another factor to be taken into account is the impact of fission product release due to failed fuel elements. Fission products released from the fuel would be free to circulate throughout the system and deposit on the inner surfaces of system components. Some of the fission products of concern include Ag, As, Cs, I, Pd, Rb, Ru, Tc, Te and rare earths. These products are suspected of playing an important role in catalytic corrosion of C/C material. Further testing is needed to understand the role of fission products on C/C composite performance.
Mitigating corrosion problems with coatings: A solution to mitigate carbon corrosion is to isolate the material from the high-temperature gas by coating the composite with a refractory system. The coating must possess low permeability to corrosive species, chemical compatibility with the substrate, good adhesion without excessive penetration, and comparable CTE to avoid spallation. Also, in order to maintain recuperator efficiency, the coating system must provide good thermal conductivity.
Coating such as silicon carbide have been used extensively in the aerospace industry as a primary barrier to corrosion. SiC forms a protective SiO2 scale in pure oxygen, but problems arise in complex gas mixtures. When the oxygen potential is not sufficient, the protective SiO2 layer cannot form and weight loss occurs by decomposition or active oxidation. SiC and SiO2 may chemically react with H2O and H2 in a low oxygen potential environment according to the following reactions: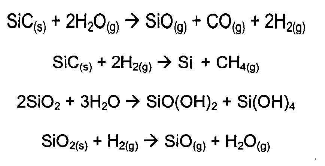
One of the principal limiting factors in the corrosion resistance of SiC coated C/C composites is the formation of crack patterns in the SiC coating as a result of the thermal expansion mismatch between substrate and coating. Clearly, open cracks provide easy paths for ingress of corrosive species and serious localized damage to the carbon-carbon substrate. At the envisioned recuperator operating temperature, the viscosity of silica glass is too high to provide crack-sealing properties. Advanced coating systems may be more effective barriers to corrosion and constitute and important area for further evaluation and development.