The CVD reactions to deposit pyrolytic graphite are based on the thermal decomposition of hydrocarbons. The most common precursor is methane, which is generally pyrolyzed at 1100C or above, over a wide range of pressure from about 100Pa to 105 Pa. The reaction in a simplified form is as follows:
Eq.(1) CH4 →C + 2H2
Other common precursors are ethylene and acetylene. Acetylene can also be decomposed at lower temperature and at pressures up to 1 atm, in the presence of a nickel catalyst. Another common precursor is propylene which decomposes in the 1000-1400C temperature range at low pressure.
The activation energies for the decomposition of these precursor gases still are not accurately known and shown a considerable scatter. The reported values are as follows:
Methane 78-106 kcal/g.mole
Ethane 60-86 kcal/g.mole
Acetylene 30-50 kcal/g.mole
Deposition mechanism: The pyrolysis of a hydrocarbon, such as shown in reaction equation (a), is actually a series of more complex reactions involving molecules of gradually increasing size. A possible mechanism of deposition of pyrolytic graphite is deduced from a series of experiments carried out in the apparatus shown in fig.7.1.
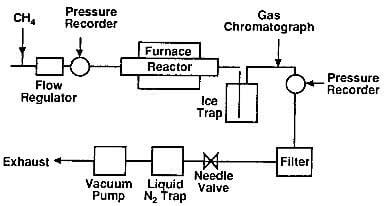
fig.7.1-schematic of experimental apparatus for the production of pyrolytic graphite
In this article, a spectrographic analysis of the by-products of the decomposition of methane revealed the presence of large amounts of acetylene, ethylene, and benzene, plus a variety of compounds consisting mostly of the polyaromatic hydrocarbons such as naphthalene, anthracene, phenantrene, acenapthylene, pyrene, and fluoranthene, in addition to the deposited pyrolytic grpahite. Some of these compounds form the soot and tar-like deposits which are often observed on the wall of CVD reactors during carbon deposition.
It is generally agreed that the following simplified deposition sequence is taking place:
Mathane →Benzene →Polyaromatic hydrocarbons →Carbon